PharmaShots Weekly Snapshots (January 22 – January 25, 2024)
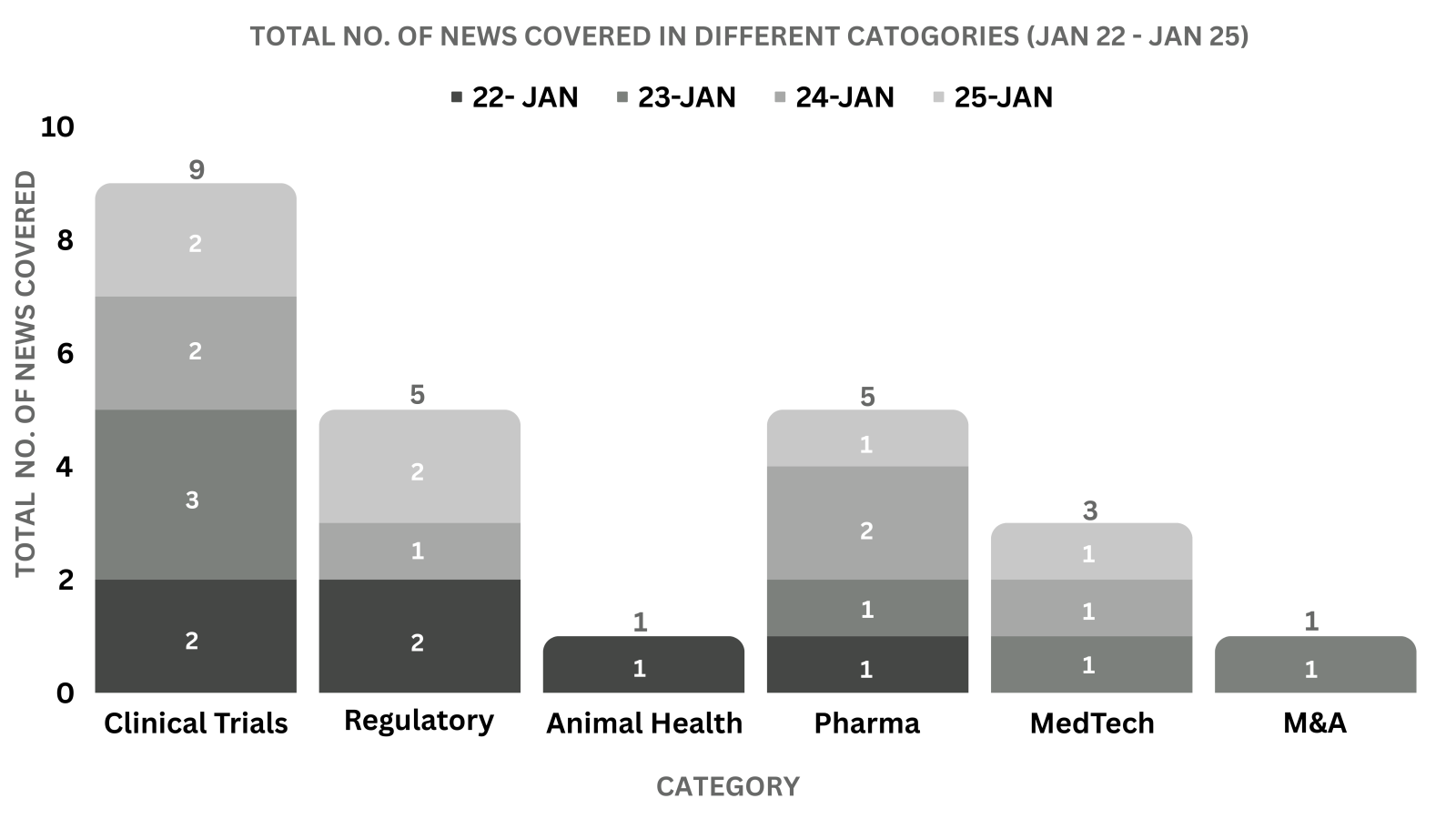
This week PharmaShots’ news was all about the updates on Regulatory, Clinical Trials,Pharma, Animal Health & MedTech. Check out our full report below:

The US FDA Grants Full Approval to Johnson & Johnson’s Balversa for the Treatment of Locally Advanced or Metastatic Urothelial Carcinoma (UC)
Read More: Johnson & Johnson
Health Canada Approves Galderma’s Restylane SHAYPE for Chin Augmentation
Read More: Galderma
The US FDA Clears Harbour BioMed’s IND Application of HBM9027 for Solid Tumors
Read More: Harbour BioMed
The US FDA Issues Complete Response Letter to Theratechnologies’ sBLA of Tesamorelin for Abdominal Fat Reduction in HIV and Lipodystrophy Patients
Read More: Theratechnologies
The USPTO Grants Patent to RedHill’s Talicia for H. Pylori Infections
Read More: RedHill

BMS Highlights Results from the P-III (CheckMate -8HW) Study of Opdivo (nivolumab) + Yervoy (ipilimumab) for Metastatic Colorectal Cancer at ASCO Gastrointestinal Cancers Symposium 2024
Read More: BMS
AstraZeneca Highlights the P-III Results for the Combination of Imfinzi, TACE, and Bevacizumab to Treat Hepatocellular Carcinoma (HCC)
Read More: AstraZeneca
BMS Highlights 4-Year Follow-Up Data from P-III Evaluation of Opdivo with Cabometyx to Treat Advanced Renal Cell Carcinoma (RCC)
Read More: BMS
BioNTech and DualityBio Initiate the P-III Evaluation of BNT323/DB-1303 in Metastatic Breast Cancer
Read More: BioNTech & DualityBio
Ionis Highlights Results from the P-III (OASIS-HAE) Study of Donidalorsen for Treating Hereditary Angioedema
Read More: Ionis
Eli Lilly Reports Data from the P-I/II (AK-OTOF-101) Trial of AK-OTOF for Genetic Hearing Loss
Read More: Eli Lilly
PharmAbcine Initiated P-Ia/b Study by Dosing First Patient with PMC-309 for Treating Advanced or Metastatic Solid Tumors
Read More: PharmAbcine
Ferring Pharmaceutical Highlights the Updates on its Real-World Evidence Study for Adstiladrin in Bladder Cancer
Read More: Ferring Pharmaceutical
Onward Therapeutics Highlights the P-I Trial Updates of OT-A201 for the Treatment of Solid Tumors
Read More: Onward Therapeutics

Coherus BioSciences Reports the Divestiture of its Ophthalmology Franchise to Sandoz for $170M Up Front
Read More: Coherus BioSciences & Sandoz
Arcutis Introduces Zoryve (roflumilast) Topical Foam, 0.3%, for Treating Seborrheic Dermatitis in the US
Read More: Arcutis
GenEdit and Genentech Collaborate to Discover and Develop Novel Nanoparticles for Autoimmune Diseases
Read More: GenEdit & Genentech
EraCal Therapeutics Signs a Collaboration and License Agreement with Novo Nordisk to Develop and Commercialize its Small Molecule Program
Read More: EraCal Therapeutics & Novo Nordisk
Elegen and GSK Sign a Collaboration Agreement to Develop Elegen’s Cell-Free DNA Production Technology
Read More: Elegen & GSK

Veterinary Pharmaceutical Solutions to Acquire Diamond Animal Health
Read More: Veterinary Pharmaceutical Solutions & Diamond Animal Health

Pi-Cardia’s ShortCut has been Granted with Breakthrough Device Designation from the US FDA
Read More: Pi-Cardia
Bionano Introduces Stratys System for High Throughput Optical Genome Mapping (OGM)
Read More: Bionano
AbSolutions Med’s Obtains US FDA’s Breakthrough Device Designation for Rebuild Bioabsorbable Abdominal Wall Closure Device for Incisional Hernia
Read More: AbSolutions Med

For an Aggregate of $2.2B, Sanofi Acquires Inhibrx Thereby Adding INBRX-101 for Alpha-1 Antitrypsin Deficiency (AATD) to its Pipeline
Read More: Sanofi & Inhibrx
Related Post:- PharmaShots Weekly Snapshots (January 15 – January 19, 2024)
Tags

Shivani was a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She was covering news related to Product approvals, clinical trial results, and updates. We can be contacted at connect@pharmashots.com.